Asymmetric Synthesis Using Acylammonium Salts
Although acylammonium salts are well-studied, chiral α,β-unsaturated acylammonium salts have received much less attention. While these intermediates are convenient synthons, are readily available from several commodity unsaturated acids and acid chlorides, and possess three reactive sites, their application in organic synthesis has been limited because of the lack of appropriate chiral Lewis bases for their generation. In recent years, the utility of chiral, unsaturated acylammonium salts has expanded considerably, thus demonstrating the unique reactivity of this intermediate leading to the development ofadiverse array of catalytic, asymmetric transformations including organocascade processes. Our group first used these reactive intermediates in the synthesis of pyrrolidinone subunits. We envisioned that a suitable Michael donor bearing a pendant amine, for example α- or β-aminomalonates, could serve as a bis-nucleophile to undergo an enantioselective nucleophile-catalyzed Michael/proton transfer/lactamization (NCMPL) cascade with the α,β-unsaturated acylammonium D. We described the first highly enantioselective version of this cascade process with commodity acid chlorides using readily available O-trimethylsilylquinidine (TMSQD), thus leading to various optically active nitrogen heterocycles. We further demonstrated the utility and practicality of this organocascade process by application to a concise, scalable synthesis of a key pyroglutamic acid intermediate for an FDA-approved drug and its broader use for the syntheses of piperidinones, enol lactones, and dihydropyridones.
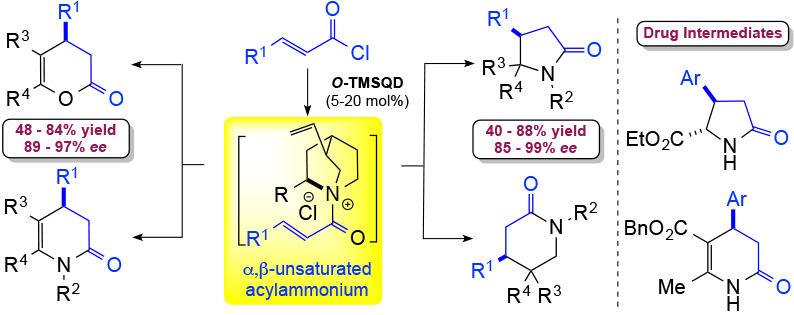
1) Direct Catalytic Asymmetric Synthesis of N-Heterocycles from Commodity Acid Chlorides Employing α,β-Unsaturated Acylammoniums Vellalath, S.; Van, K. N.; Romo, D. Angew. Chem. Int. Ed., 2013, 52, 13688–13693.
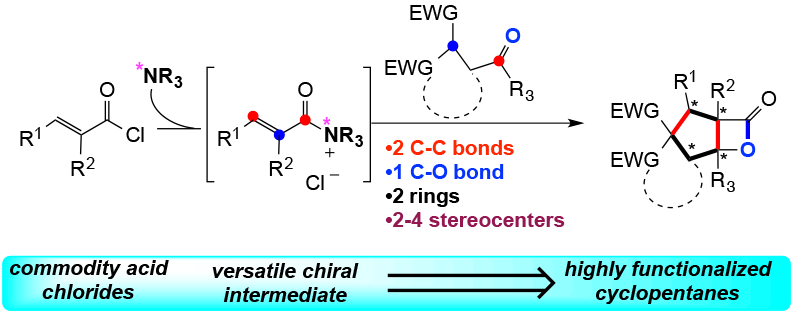
Building on our previously described aldol-β-lactonization process we were attracted to the potential utility of chiral α,β-unsaturated acylammonium intermediates given the presence of three disparate reactive sites that could be revealed sequentially to induce tandem bond-forming events. We anticipated generation of the unsaturated acylammonium through catalytic, asymmetric acti- vation of acid chlorides by a chiral amine nucleophile. Following Michael addition by an appropriate nucleophile, a chiral ammonium enolate is revealed, enabling a-addition of an electrophile to deliver the acylammonium, and regeneration of the amine catalyst by acyl substitution with a second nucleophile would finally deliver the triply functionalized adduct. We applied this process for the synthesis of complex cyclopentanes utilizing chiral a,b-unsaturated acylammonium intermediates, readily generated by activation of commodity unsaturated acid chlorides with chiral isothiourea catalysts. This efficient methodology enables the construction of two C–C bonds, one C–O bond, two rings and up to three contiguous stereogenic centres delivering complex cyclopentanes with high levels of relative and absolute stereocontrol.
2) Rapid Assembly of Complex Cyclopentanes Employing Chiral, α,β-Unsaturated Acylammonium Intermediates Liu, G.; Shirley, M. E.; Van, K. N.; McFarlin, R. L.; Romo, D. Nature Chem., 2013, 5, 1049-1057.
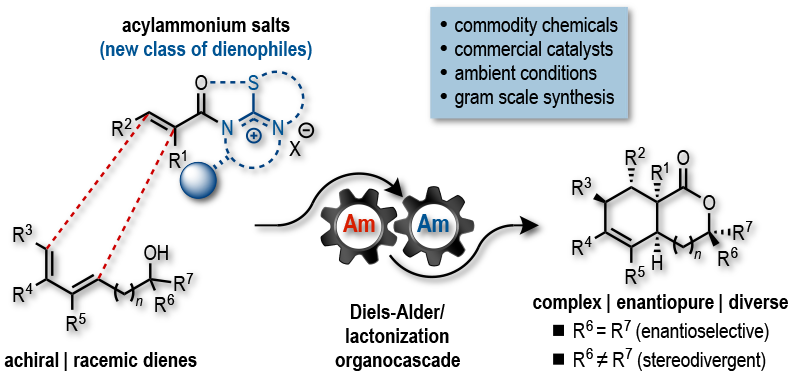
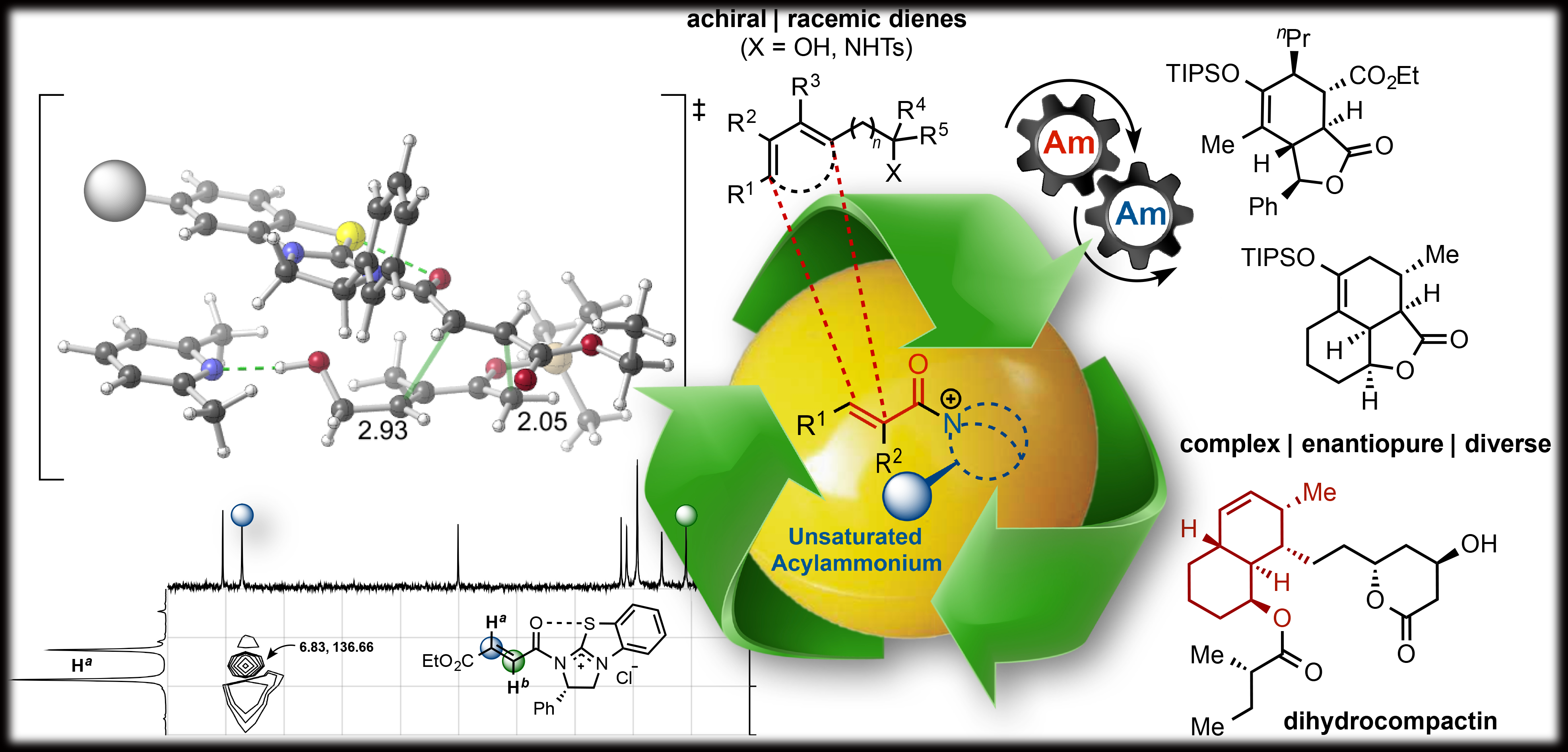
Inspired by the results of our previous work, we sought to explore the reactivity of α,β-unsaturated acylammonium salts as dienophiles in Diels-Alder reactions anticipating that these intermediates might emulate the electronic properties of activated dienophiles. To test the reactivity of α,β-unsaturated acylammonium salts as dienophiles, we targeted the synthesis of cis- and trans-fused bicyclic γ- and δ-lactones which are ubiquitous structural motifs found in bioactive terpenoids and pharmaceuticals. We envisioned that this bicyclic architecture could be constructed in a single operation by a Diels–Alder/lactonization cascade between acylammonium salts, generated in situ from acid chlorides or carboxylic acids (activated in situ) , a chiral tertiary amine organocatalyst, and rationally designed dienes. We found that these α,β-unsaturated acylammonium salts undergo a highly diastereo- and enantioselective Diels–Alder/lactonization organocascade that generates cis- and trans-fused bicyclic γ- and δ-lactones bearing up to four contiguous stereocenters. Moreover, we described the first examples of DA-initiated, stereodivergent organocascades delivering complex scaffolds found in bioactive compounds. The origins of stereoselectivity were rationalized through computational studies. In addition, we demonstrated the utility of this methodology is demonstrated through a concise approach to the core structure of glaciolide and formal syntheses of fraxinellone, trisporic acids, and trisporols.
3) Acylammonium Salts as Dienophiles in Diels-Alder/Lactonization Organocascades Abbasov, M. E.; Hudson, B. M.; Tantillo, D. J.; Romo D. J. Am. Chem. Soc. 2014, 136, 4492–4495.
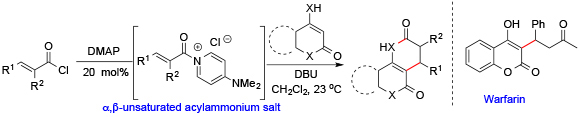
Building on these studies, we sought to explore the use of cyclic 1,3-dicarbonyls and enamino ketones to access polycyclic dihydropyranones and dihydropyridones using unsaturated acylammonium salts. Dihydropyranones (enol lactones) are frequently encountered structural sub-units in natural products and bioactive compounds. For instance, these heterocycles constitute the basic skeleton of the neoflavonoid, coumarin, and iridoide family of natural products. They are also versatile intermediates for the synthesis of 2-pyranones, γ-lactones, cyclic enamines, and substituted benzenoids. Established methods to synthesize these molecules suffer from harsh reaction conditions. We thus developed a mild, direct synthesis of polycyclic dihydropyranones and a dihydropyridone from acid chlorides via α,β-unsaturated acylammonium salts and cyclic 1,3-dicarbonyl compounds, promoted by 4-dimethylaminopyridine (DMAP). The utility of this methodology was demonstrated in the formal synthesis of the alkaloid dielsine and the anticoagulant warfarin. Comparative 13C NMR studies of acylammonium salt intermediates also provided insights into the impact of their formation on the reactivity of the β-carbon.
4) Utility and NMR Studies of α,β-Unsaturated Acylammonium Salts: Synthesis of Polycyclic Dihydropyranones and a Dihydropyridone Vellalath, S.; Van, K. N.; Romo, D. Tetrahedron Lett. 2015, 23, 3647–3652.
Further, we described additional examples of stereodivergent Diels-Alder/lactonization processes leading to complex, polycyclic adducts, new applications toward formal syntheses of natural products or core structures, and the potential of this reaction for diversity-oriented synthesis through diastereodivergent Diels-Alder/lactonization processes. The latter is made possible by the discovery that diastereoselectivity of the Diels-Alder/lactonization can be altered by judicious choice of Brønsted-base additive. Finally, we provided evidence that these organocascades may be rare examples of reactions in which diastereoselectivity is entropy-driven.
5) Stereodivergent, Diels‐Alder‐Initiated Organocascades Employing α,β-Unsaturated Acylammonium Salts: Scope, Mechanism, and Applications Abbasov, M. E.; Hudson, B. M.; Tantillo, D. J.; Romo D., Chem. Sci. 2017, 8, 1511-1524.
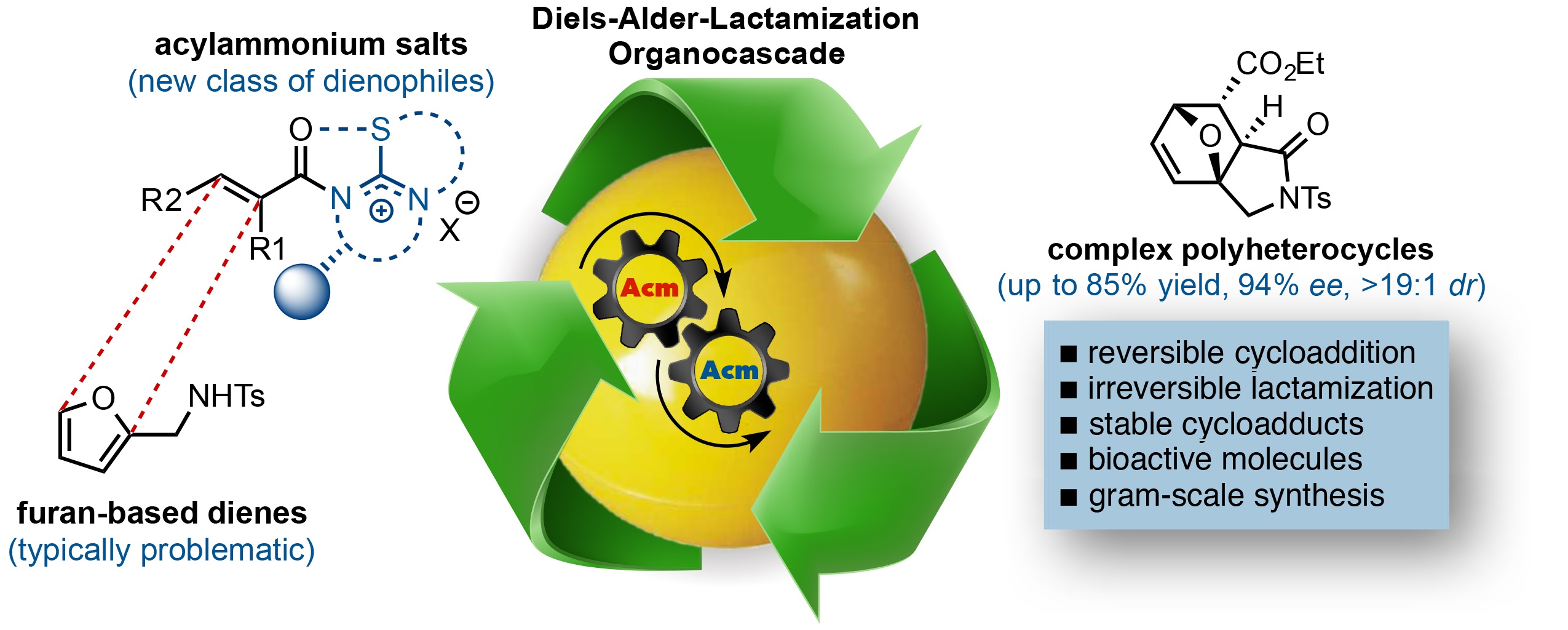
More recently, we applied our α,β-Unsaturated Acylammonium salt methods to the Diels-Alder reaction using furans as dienes. Due to their aromaticity, furans are often unreactive and undergo reversible Diels-Alder cycloadditions, thus strong Lewis acids or high pressure are typically required to promote these reactions. We found that the termination step of a Diels-Alder/lactamization organocascade can provide a solution to the reversibility of these cycloadditions with furanyl dienes by careful choice of a suitable pendant terminating nucleophile. The use of furan-based dienes bearing pendant sulfonamides lead to the generation of oxa-bridged, trans-fused tricyclic γ-lactams. This process constitutes the first highly enantio- and diastereoselective, organocatalytic Diels-Alder cycloadditions with these typically problematic dienes due to their reversibility. Computational studies also suggest that the high diastereoselectivity with these furan dienes may be due to a reversible Diels-Alder cycloaddition for the endo adducts. In addition, the utility of this methodology was demonstrated through a concise approach to a core structure with similarity to the natural product isatisine A and a nonpeptidyl ghrelin-receptor inverse agonist.
6) Enantioselective Diels-Alder-Lactamization Organocascades Employing a Furan-Based Diene Abbasov, M. E.; Hudson, B. M.; Kong, W.; Tantillo, D. J.; Romo. D. Org. Biomol. Chem. 2017, 33, 592-594.